Immediate Test Change
Updates to H. pylori Urea Breath Test (HPYLBR)
Updated Test
Helicobacter pylori Breath Test (HPYLBR)
Effective May 2, 2023, the Immunopathology Laboratory section of Cleveland Clinic Laboratories will provide a new H. pylori urea breath test.
The qualitative test, based on infrared spectroscopy using 13C, is an aid in diagnosing active infection with Helicobacter pylori and post-treatment monitoring in adult and pediatric patients ages 3-17 years old.
Changes to Specimen Collection
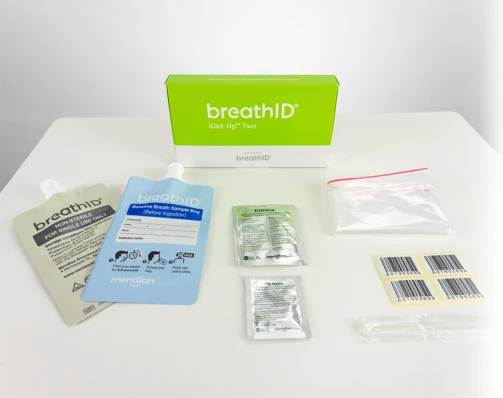
Collection Kits
The BreathID® Hp® Two test requires specimen collection with the BreathID® IDkit Hp® Two kit.
One box contains five kits. Each kit contains:
- (1) Blue baseline breath collection bag
- (1) Grey post-ingestion breath collection bag
- 75mg 13C-urea tablet
- 4.3g package of Citrica (citric acid)
- Straw
- Drinking cup
- User guide
- (4) Labels
- Sample transport bag
- Package insert
Test Overview
Test Name
Helicobacter pylori Breath Test
Test Code
CPT Code
83013
Methodology
13C Infrared Spectroscopy
Specimen Type
1 baseline (blue) and 1 post-dose (gray) breath sample bag collected with a BreathID® IDkit Hp® One kit
Stability
Ambient
14 days
Refrigerated
Unacceptable
Frozen
Unacceptable
Reference Range
Negative
Clinical Information
The Urea Breath Test is used as an aid in the diagnosis of current infection with Helicobacter pylori.
Limitations
Despite very high specificity, false positive results may occur due to other gastric organisms, such as H. heilmanni, as well as in patients with hypo- or achlorhydria.
False-negative results may occur in patients who have received antibiotics, proton pump inhibitors, or bismuth preparations.
Clinical correlation is required.
