Special Communication
Supply Shortages Impacting Gonorrhea, Chlamydia, and Trichomoniasis Testing
As a result of nationwide supply shortages caused by COVID-19 demands, Cleveland Clinic Laboratories may periodically be unable to provide Aptima® Urine Specimen Collection Transport Tubes for gonorrhea, chlamydia, and trichomoniasis testing.
Aptima tubes will continue to remain an acceptable specimen collection container for testing.
Because of the severe shortage of urine tubes, providers should reserve urine testing for men with persistent urethritis. This practice aligns with guidance from the Centers for Disease Control and Prevention (CDC).

What Tests Are Affected
GC/Chlamydia Amplification, Urine (UGCCT)
Ordering Options for Gonorrhea/Chlamydia Testing
- GC/Chlamydia Amplification, Urine (UGCCT) using self-supplied Aptima Urine Specimen Collection Transport Tubes
- GC/Chlamydia Amplification, Genital, Rectal and Oral Specimens (GCCT) using an Aptima Swab Kit
Trichomonas vaginalis Amplification (TRVAMP)
Ordering Options for Trichomoniasis Testing
- Trichomonas vaginalis Amplification (TRVAMP) using an Aptima Swab Kit
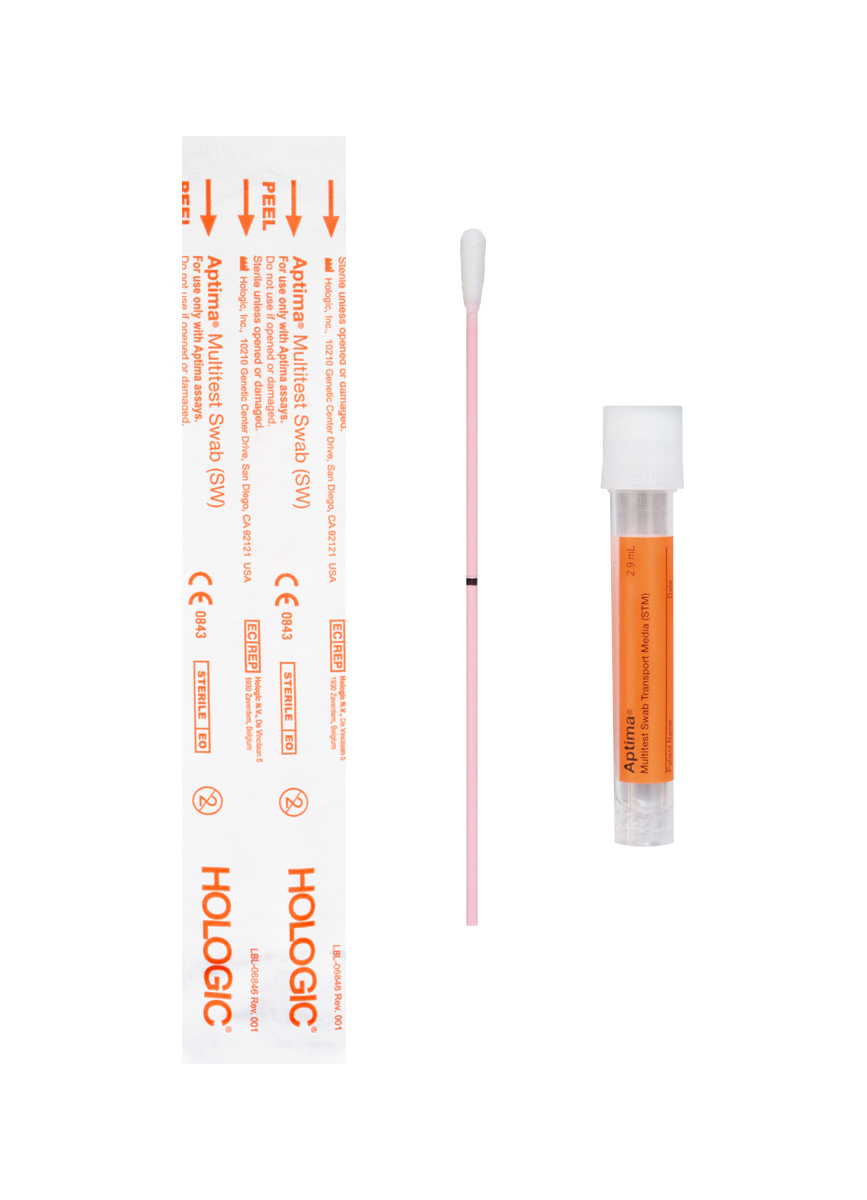
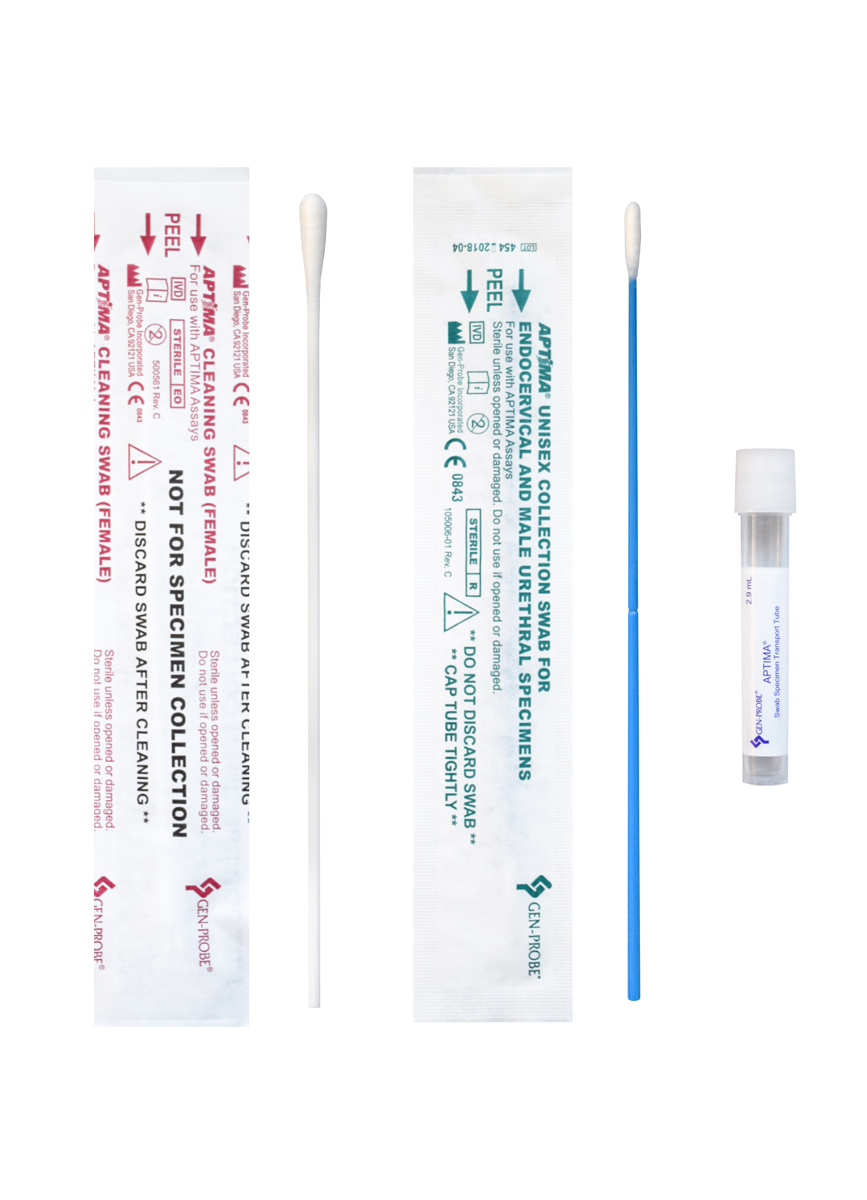
Specimen Collection for GCCT & TRVAMP
Samples can continue to be collected and submitted with either Aptima kit shown below.
Although CCL cannot provide this item, Aptima tubes will continue to remain an acceptable specimen collection container for testing.
Unacceptable Specimen Containers
Because of the limited stability of unpreserved urine for gonorrhea, chlamydia, and trichomoniasis testing, Cleveland Clinic Laboratories is currently unable to accept urine in a sterile container (i.e., specimen cup) for UGCCT and TRVAMP orders.

Other Considerations
Cleveland Clinic Laboratories will notify clients when Aptima urine tubes are back in stock and available to order. Conservative ordering practices may be necessary until supplies stabilize.
For help in prioritizing which patients to test for these pathogens, please refer to the recently-issued considerations from the CDC.