Special Communication
July 2022: Changes to Sulfonylurea Hypoglycemics (SULFON)
Effective July 5, 2022
Sulfonylurea Hypoglycemics (SULFON)
Changes to Specimen Requirements
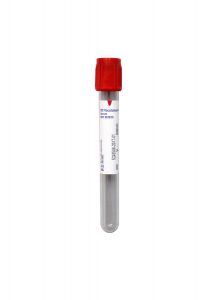
Specimen Type:
Serum
Volume:
1 mL
Minimum Volume:
0.3 mL
Collection Container:
Red (Serum) No Additive Tube
Transport Temperature:
Frozen
Additional Info:
Centrifuge and aliquot ASAP or within 2 hours of collection.
Separate specimens must be submitted when multiple tests are ordered.
Alternative Specimen
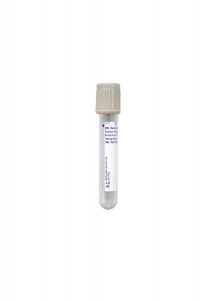
Specimen Type:
Plasma
Volume:
1 mL
Minimum Volume:
0.3 mL
Collection Container:
Grey Hemogard, Sodium Fluoride/Potassium Oxalate Tube
Transport Temperature:
Frozen
Additional Info:
Centrifuge and aliquot ASAP or within 2 hours of collection.
Separate specimens must be submitted when multiple tests are ordered.
Changes to Reference Ranges
Refer to Report
- Rosiglitazone
- Chlorpropamide
- Glimepiride
- Glipizide
- Pioglitazone
- Glyburide
- Nateglinide
- Repaglinide
- Tolazamide
- Tolbutamide
Changes to Test Build
Add:
- Rosiglitazone
- Pioglitazone
Remove:
- Acetohexamide
