Special Communication
December 2022: Changes to Acceptable Specimen Containers for Azole Anti-Fungal Therapeutic Drug Monitoring
Effective December 20, 2022
Plasma collected in Green Lithium Heparin Tubes will no longer be accepted for azole anti-fungal therapeutic drug monitoring tests.
Please collect samples for Azole Anti-Fungal Therapeutic Drug Monitoring tests in Red (serum) No Additive Tubes, centrifuge, and transfer the serum to an aliquot tube.
Tests Affected:
- Fluconazole (FLUC)
- Isavuconazole (ISACON)
- Itraconazole (ITRAC)
- Posaconazole (POSACN)
- Voriconazole (VORCON)
Updated Specimen Requirements
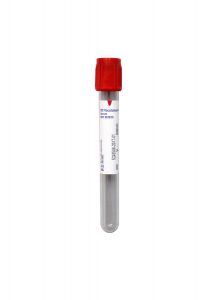
Specimen Type:
Serum
Volume:
0.5 mL
Collection Container:
Red (Serum) No Additive Tube
Transport Temperature:
Refrigerated
