Immediate Test Changes
Changes to Acceptable Clobazam (CLOBAZ) Specimen Types
Specimen Requirements
Volume:
0.5 mL, serum
Minimum Volume:
0.35 mL, serum
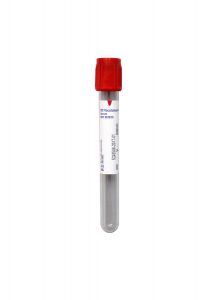
Collection Container:
Red BD Hemogard™ Serum Tube (No Additive)
Specimen Instructions:
Draw specimen immediately before the next scheduled dose. Trough specimens are recommended, as therapeutic ranges are based on trough collections.
Centrifuge the specimen within two hours of collection, transfer the serum into a standard plastic aliquot tube, and transport refrigerated.
Transport Temperature:
Refrigerated
Any additional information will be included in a future Technical Update. If you have any questions about these changes, please contact Client Services for assistance.