Specimen Collection & Transport
Requirements
Safe, effective testing requires specimens to be appropriately collected, labeled, and documented.
Review the information outlined below for details.
For specific test information, please refer to the Test Directory. If you have additional questions, contact Client Services.
Specimen Collection & Transport
Specimen Requirements
For specific test information, please refer to the Test Directory.
Specimens are only acceptable for processing when collected and submitted appropriately.
If you have additional questions, contact Client Services.
The Test Directory outlines:
- Full test names & ordering mnemonics
- CPT codes
- Performing laboratory
- Specimen requirements
- Stability
- Test methodology
- Days performed & reported
- Values & interpretation
- Clinical overview
- Supporting documentation
Labeling Requirements
All specimen containers and requisitions must be labeled with:
- Patient’s Full Name
- Date of Birth
- Specimen Type
- Collection Date
Testing will not be performed and the client will be notified if:
- Identifiers on the container do not match those listed on the requisition.
- The specimen is inappropriately or incompletely labeled.
Proper labeling ensures accurate identification and optimum integrity of specimens from the time of collection until completion of testing.
Documentation Requirements
A test requisition must accompany specimens submitted for testing.
For manual orders – Each section on the Test Requisition must be filled out, including:
- Patient Identifiers: Full name & date of birth (DOB)
- Insurance / Billing Information
- Clinical Information
- Ordering Provider Contact Details
- Specimen Information
- Selected test(s)
Test Requisition
A test requisition is a form required to accompany specimens sent to the laboratories for testing.
The patient identifiers on the specimen container must match those provided on the test requisition.

Filling Out a Test Requisition
For Manual Orders: A test requisition is a form required to accompany specimens sent to the laboratories for testing.
Please provide the required information in each section, then prepare the requisition and specimen for transport.

Patient Information
The following patient information is required:
- Patient’s Full Name
- Date of Birth
- Specimen Type
- Collection Date
Insurance Billing Information
Please provide all of the patient’s insurance information or attach a scanned copy of their insurance card.
If you do not have a payable code or a responsible party signature, federal law states that we must bill your account for the performed testing.
Client Information
Please ensure that the following information is provided:
Facility/Ordering Location
- Name & client ID
- Address
Ordering Physician
- First & last name
- Contact information
Requisitions:
Multiple Specimens
Specimens are occasionally sent to different areas of the lab for testing. Therefore, accompanying documentation is needed for each specimen as it is processed.
Example: If a patient had blood drawn for a CBC and a urine sample collected for culture, the blood specimen and the urine specimen would each require a separate requisition.
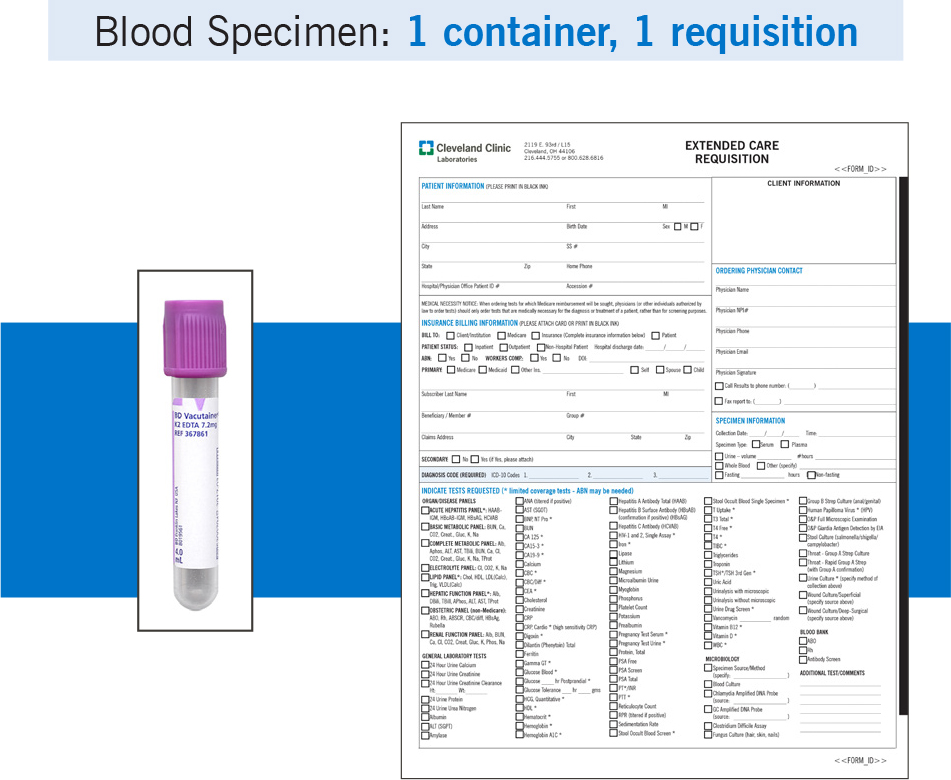
Blood specimen: 1 container, 1 requisition – same patient
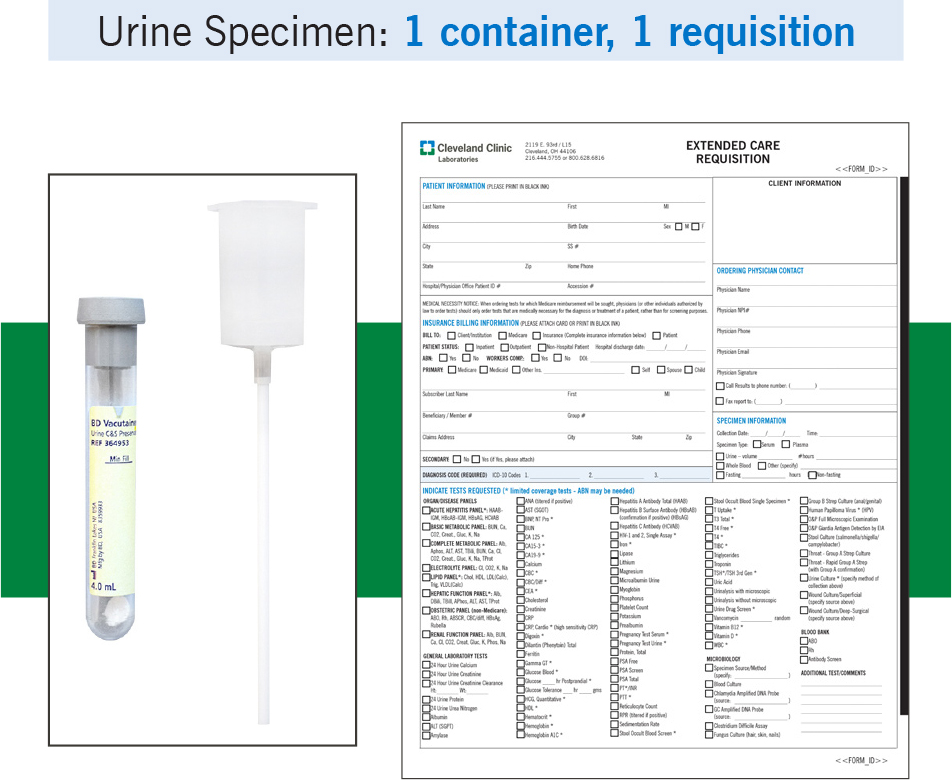
Urine specimen: 1 container, 1 requisition – same patient
Requisitions:
Multiple Tests
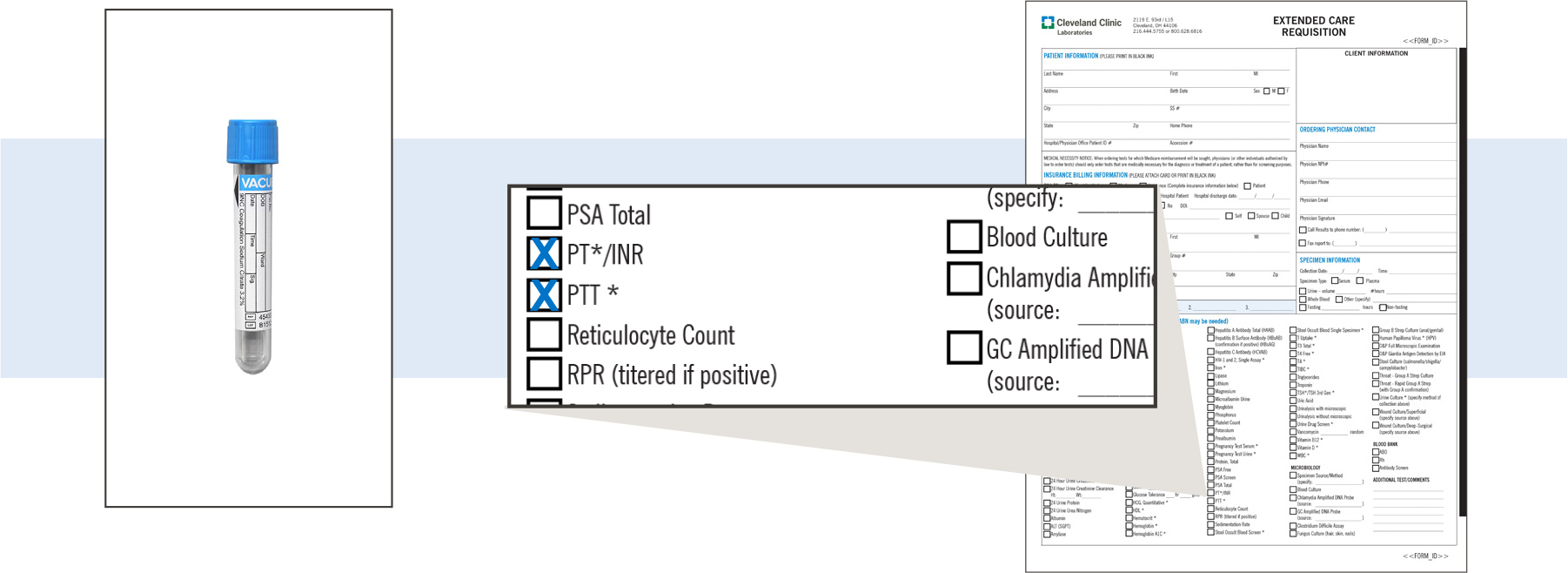
If several tests are to be performed on a single specimen, the tests can be listed on the same requisition.
Example: A blood specimen could have both a PT/INR and PTT indicated on a single requisition.
Rejection Criteria
Specimens will be rejected under the following conditions:
Documentation-related
- Mismatched patient identifiers (i.e.: the two unique identifiers on the specimen container do not match those on requisition or electronic order).
- Unlabeled or incompletely labeled.
- Specimen submitted without a written or electronic order.
Specimen-related
- Improper specimen transport temperature.
- Incorrect specimen type submitted for the requested test.
- Insufficient volume/quantity not sufficient (QNS).
Container-related
Submitted in an unacceptable container type, including:
- A syringe with a needle attached
- Broken
- Glass
- Leaking
Test-dependent
- Hemolysis.
- Unacceptable specimen age.
