Test Name
Anti-Platelet Factor 4 (PLATF4)
CPT Codes
86022 (x2)
Methodology
Enzyme-Linked Immunosorbent Assay (ELISA)
Turnaround Time
1 day
Specimen Requirements
Volume:
2 mL
Minimum Volume:
1 mL
Specimen Type:
Plasma
Collection Container:
Light Blue Sodium Citrate Coagulation Tube
Transport Temperature:
Froze
Stability
Ambient:
4 hours
Refrigerated:
Unacceptable
Frozen:
2 months
Additional Information
Background Information
Heparin-induced thrombocytopenia (HIT) is a clinically significant immune-mediated disorder, characterized by antibodies forming immune complexes with the chemokine platelet factor 4 (PF4) bound to unfractionated heparin (UFH), leading to paradoxical thrombosis.1 The IgG immune complexes engage Fc-gamma receptor IIa (FcγRIIa) expressed on platelets and possibly leukocytes,2 initiating a signal transduction cascade resulting in cellular activation. Activated platelets may potentiate thrombin generation, form thrombi, and induce a prothrombotic state involving both the venous and arterial systems.3 In the process, platelets are consumed, which leads to the observed thrombocytopenia. It is critical that HIT is recognized early so that patients can be alternatively anticoagulated and avoid further exposure to heparin.
Based on the findings of a consensus report,4 HIT is regarded as a clinicopathologic syndrome, requiring both clinical features and laboratory detection of the pathologic antibodies. Clinically, HIT needs to be differentiated from other potential causes of thrombocytopenia, particularly the well-characterized non-immune heparin-associated thrombocytopenia (HAT).
A useful clinical scoring system, referred to as the “4Ts,” is helpful in determining whether a patient fits into the “high probability,” “intermediate probability,” or “low probability” category.5
The criteria below should be assessed to determine the pretest probability of HIT:
4Ts Scoring System for Assessing Clinical Risk of HIT
Score each category, then add all points to determine maximum score. (Maximum possible score = 8 points)
Thrombocytopenia
0 points = <30% fall or nadir <10×109/L
1 point = 30-50% fall or nadir 10-19×109/L
2 points = >50% fall and nadir 20-100×109/L
Timing of Platelet Count Fall
0 points = <4 days without recent exposure
1 point = Consistent with day 5-10 (but not clear), or >10 days, or ≤1 day with heparin 30-100 days prior
2 points = Day 5-10 or ≤1 day if recent heparin (within 30 days)
Thrombosis
0 points = None
1 point = Progressive, recurrent, or silent thromboses
2 points = Proven thrombosis, skin necrosis, or acute systemic reaction with heparin bolus
Other Causes for Thrombocytopenia
0 points = Definite
1 point = Possible
2 points = None evident
Pretest Probability
<3 points = Low
4-5 points = Intermediate
6-8 points = High
Clinical Indications
Suspicion for HIT in patients exposed to UFH or low molecular-weight heparin (LMWH).
Interpretation
Testing for HIT encompasses both immunologic (Anti-PF4 IgG ELISA) & functional assays (heparin-induced platelet aggression [HIPA] and serotonin release [SRA]).
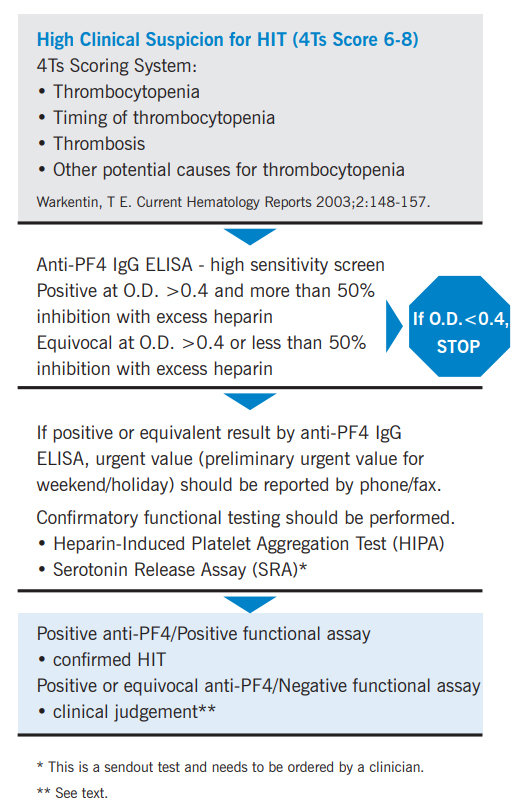
Positive Anti-PF4 IgG ELISA Result
• When the anti-PF4 IgG antibody ELISA and platelet functional assay (heparin-induced platelet aggregation testing [HIPA] or serotonin release assay [SRA]) are both positive, the diagnosis of HIT is supported by laboratory findings.
• If the anti-PF4 IgG antibody ELISA is positive or equivalent, but the platelet functional assays (see Methodology) are both negative, the diagnosis of HIT is less likely. However, clinical judgement is important since the confirmatory tests are not as sensitive for detecting HIT as the ELISA. Reassessing the pretest probability is suggested. If clinical suspicion remains high, retesting may be justified in case the antibody titer was too low to induce immune complex-mediated platelet activation in the initial work-up.
• If the anti-PF4 IgG antibody ELISA is positive, but the HIPA is negative, ordering the SRA is suggested if clinical suspicion remains high. The SRA has a higher sensitivity than the HIPA.
Negative Anti-PF4 IgG ELISA Result
• If the anti-PF4 IgG antibody ELISA is negative, HIT is very unlikely and further testing is unnecessary. In rare situations where clinical necessity dictates confirmatory testing despite a negative anti-PF4 result, the SRA is the recommended confirmatory assay since it has a higher sensitivity than the HIPA.
Limitations
A positive reaction obtained by the anti-PF4 IgG antibody screening ELISA does not confirm the diagnosis of HIT; a functional assay must be performed to confirm HIT.
The presence of immune complexes or other immunoglobulin aggregates in the patient sample may cause nonspecific binding in the ELISA and produce false positives.
The platelet functional assays are not as sensitive as the anti-PF4 IgG antibody ELISA, but have higher specificity and, therefore, should be used only as confirmatory tests.
Non-heparin dependent antibodies, such as anti-HLA, can cause platelet activation independent of heparin/PF4 immune complexes, rendering the functional assays indeterminate.
Methodology
Laboratory testing for HIT is a two-stage process. Testing begins with a high-sensitivity ELISA screen for anti-PF4 IgG antibodies.
No Antibodies Detected:
If the O.D. < 0.4, a diagnosis of HIT is unlikely (negative predictive value 97-99%), and no further testing is necessary.
Heparin Dependent Anti-PF4 IgG Antibodies are Present:
If the O.D. > 0.4 and % inhibition with soluble heparin is > 50%, the assay is positive. The ordering clinician is immediately notified of the result.
The HIPA platelet functional assay (with higher specificity) is then performed to confirm the diagnosis of HIT. Recommendations to order the SRA will be made.
Heparin-Dependent Anti-PF4 IgG Antibodies Equivocal:
If the O.D. > 0.4 and % inhibition with soluble heparin is < 50%, the assay is equivocal.
Platelet functional assays (with higher specificity) are performed to further evaluate.
The anti-PF4 IgG assay (GTI Diagnostics, Waukesha, WI) is performed by a solid-phase ELISA method. The antibody in the patient’s specimen will bind to microwells coated with PF4 complexed with polyvinyl sulfonate. After serial addition of alkaline phosphatase labeled anti-human IgG and the substrate p-nitrophenyl phosphate, the optical density (O.D.) of developed color is measured in a spectrophotometer. The anti-PF4 IgG assay is performed with the patient’s specimen with and without additional soluble heparin (final heparin concentration of 100 U/mL) as well as positive and negative controls.
If the O.D. is less than 0.4, it is considered a negative result.
If the anti-PF4 IgG result is positive with the O.D. greater than 0.4, % inhibition will be calculated as follows:
Patient specimen with heparin – Negative control
[1- (___________________________ )] x 100 = % Inhibition
Patient specimen without heparin – Negative control
The inhibition of a positive reaction by 50% or more (by calculation) in the presence of excess heparin is considered confirmatory for the presence of specific antibodies that react with PF4: heparin.
Heparin-induced platelet aggregation testing (HIPA) is performed by mixing donor platelet-rich plasma (providing the platelets) with patient plasma (providing the antibodies). Low-dose UFH (0.1-0.5 U/ml) is added. If antibodies are present, immune complexes form. The antibody/PF4/heparin complexes activate the platelet FcγRIIa and the platelets aggregate (> 30% aggregation indicates a positive confirmatory result). As an additional control for specificity, high-dose UFH (100 U/ml) is also tested. The excess heparin prevents the formation of immune complexes and should not induce platelet aggregation. If aggregation is seen with the high-dose heparin, the test is considered indeterminate due to non-specific cross reactivity. This test has a higher specificity for pathologic HIT than the screening assay, but the sensitivity is only 50-80%.
The serotonin release assay (SRA) (sent out) and other washed platelet assays offer more sensitive confirmatory testing (80-90%). These tests are performed in a similar manner to the HIPA, relying on donor platelets and patient plasma or serum. With the SRA, a > 20% release of serotonin in the presence of low-dose UHF is considered positive. If there is > 20% serotonin release in the presence of high-dose UFH, the test is considered indeterminate. If the screening test is positive with a high pretest probability, and the HIPA is negative, performing the SRA may be helpful.
In patients suspected of developing a HIT-like syndrome while on low molecular weight heparin (LMWH), confirmatory testing is performed with LMWH instead of UFH.
References
1. Kelton JG, Warkentin TE. Heparin-induced thrombocytopenia: a historical perspective. Blood. 2008 Oct 1;112(7):2607-16.
2. Xiao Z, Visentin GP, Dayananda KM, Neelamegham S. Immune complexes formed following the binding of antiplatelet factor 4 (CXCL4) antibodies to CXCL4 stimulate human neutrophil activation and cell adhesion. Blood. 2008 Aug 15;112(4):1091-100.
3. Walenga JM, Jeske WP, Messmore HL. Mechanisms of venous and arterial thrombosis in heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2000 Nov;10 Suppl 1:13-20.
4. Warkentin TE, Chong BH, Greinacher A. Heparin-induced thrombocytopenia: towards consensus. Thromb Haemost. 1998;79:1-7.
5. Warkentin TE, Heddle NM. Laboratory Diagnosis of Immune Heparin-Induced Thrombocytopenia. Current Hematology Reports. 2003;2:148-157.