Special Communication
August 2022: Changes to C Telopeptide, Beta Cross Linked (CTELO)
Effective September 6, 2022
C Telopeptide, Beta Cross Linked (CTELO)
Changes to Specimen Requirements
Specimen Type:
Serum
Volume:
1 mL
Minimum Volume:
0.5 mL
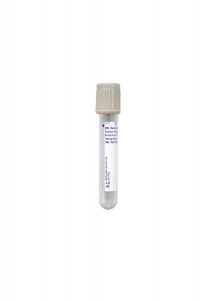
Collection Container:
Gold Serum Separation Tube (SST)
Transport Temperature:
Frozen, critical
Collection Information:
Allow the tube to sit for 15-20 minutes at room temperature to form a clot. Centrifuge and separate serum from cells ASAP or within 2 hours of collection. Transfer serum to a standard aliquot tube.
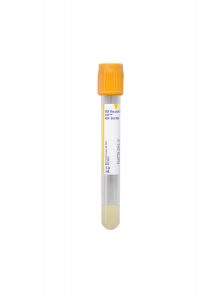
Alternative Specimen
Alternative Specimen Type:
Plasma
Volume:
1 mL
Minimum Volume:
0.5 mL
Collection Container:
Lavender K2EDTA Tube
Transport Temperature:
Frozen, critical
Collection Information:
Centrifuge and separate plasma from cells ASAP or within 2 hours of collection. Transfer plasma to a standard aliquot tube.

Changes to Reference Ranges
Male
6 Months to 6 Years:
500 – 1700 pg/mL
7 to 9 Years:
522 – 1682 pg/mL
10 to 12 Years:
553 – 2071 pg/mL
13 to 15 Years:
485 – 2468 pg/mL
16 to 17 Years:
276 – 1546 pg/mL
18 to 29 Years:
238 – 1019 pg/mL
30 to 39 Years:
225 – 936 pg/mL
40 to 49 Years:
182 – 801 pg/mL
50 to 59 Years:
161 – 737 pg/mL
60 to 69 Years:
132 – 752 pg/mL
70 to 99 Years:
118 – 776 pg/mL