Category: Special Communication
May 2023: COVID-19 Testing – CPT Code Update
Immediate Test Change
COVID-19 Testing – CPT Code Update
In accordance with recent guidance provided by the Department of Health and Human Services on May 9, 2023, the CPT code associated with COVID-19 testing will change.
Beginning May 11, 2023, the CPT code is changing as a result of the end of the COVID-19 Public Health Emergency.
The new CPT code will display in the Test Directory starting May 11, 2023.
COVID-19 Testing
New CPT Code
87635 (previous: U0003)
Additional Information
For more information, please refer to HHS’s Fact Sheet: End of the COVID-19 Public Health Emergency.
April 2023: Updates to H. pylori Urea Breath Test
Immediate Test Change
Updates to H. pylori Urea Breath Test (HPYLBR)
Updated Test
Helicobacter pylori Breath Test (HPYLBR)
Effective May 2, 2023, the Immunopathology Laboratory section of Cleveland Clinic Laboratories will provide a new H. pylori urea breath test.
The qualitative test, based on infrared spectroscopy using 13C, is an aid in diagnosing active infection with Helicobacter pylori and post-treatment monitoring in adult and pediatric patients ages 3-17 years old.
Changes to Specimen Collection
Collection Kits
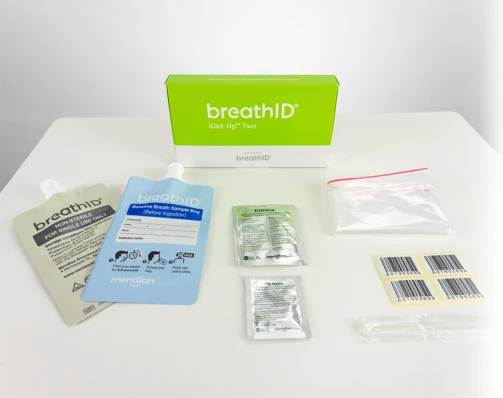
The BreathID® Hp® Two test requires specimen collection with the BreathID® IDkit Hp® Two kit.
One box contains five kits. Each kit contains:
- (1) Blue baseline breath collection bag
- (1) Grey post-ingestion breath collection bag
- 75mg 13C-urea tablet
- 4.3g package of Citrica (citric acid)
- Straw
- Drinking cup
- User guide
- (4) Labels
- Sample transport bag
- Package insert
Test Overview
Test Name
Helicobacter pylori Breath Test
Test Code
CPT Code
83013
Methodology
13C Infrared Spectroscopy
Specimen Type
1 baseline (blue) and 1 post-dose (gray) breath sample bag collected with a BreathID® IDkit Hp® One kit
Stability
Ambient
14 days
Refrigerated
Unacceptable
Frozen
Unacceptable
Reference Range
Negative
Clinical Information
The Urea Breath Test is used as an aid in the diagnosis of current infection with Helicobacter pylori.
Limitations
Despite very high specificity, false positive results may occur due to other gastric organisms, such as H. heilmanni, as well as in patients with hypo- or achlorhydria.
False-negative results may occur in patients who have received antibiotics, proton pump inhibitors, or bismuth preparations.
Clinical correlation is required.
February 2023: Send-out Test Discontinuation – T3 Uptake (T3U)
December 2022: Changes to Acceptable Tube Type for Azole Anti-Fungal Therapeutic Drug Monitoring
Special Communication
December 2022: Changes to Acceptable Specimen Containers for Azole Anti-Fungal Therapeutic Drug Monitoring
Effective December 20, 2022
Plasma collected in Green Lithium Heparin Tubes will no longer be accepted for azole anti-fungal therapeutic drug monitoring tests.
Please collect samples for Azole Anti-Fungal Therapeutic Drug Monitoring tests in Red (serum) No Additive Tubes, centrifuge, and transfer the serum to an aliquot tube.
Tests Affected:
- Fluconazole (FLUC)
- Isavuconazole (ISACON)
- Itraconazole (ITRAC)
- Posaconazole (POSACN)
- Voriconazole (VORCON)
Updated Specimen Requirements
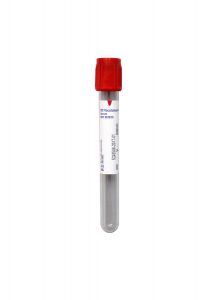
Specimen Type:
Serum
Volume:
0.5 mL
Collection Container:
Red (Serum) No Additive Tube
Transport Temperature:
Refrigerated
August 2022: Changes to C Telopeptide, Beta Cross Linked (CTELO)
Special Communication
August 2022: Changes to C Telopeptide, Beta Cross Linked (CTELO)
Effective September 6, 2022
C Telopeptide, Beta Cross Linked (CTELO)
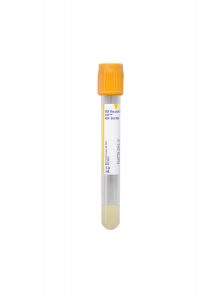
Changes to Specimen Requirements
Specimen Type:
Serum
Volume:
1 mL
Minimum Volume:
0.5 mL
Collection Container:
Gold Serum Separation Tube (SST)
Transport Temperature:
Frozen, critical
Collection Information:
Allow the tube to sit for 15-20 minutes at room temperature to form a clot. Centrifuge and separate serum from cells ASAP or within 2 hours of collection. Transfer serum to a standard aliquot tube.
Alternative Specimen
Alternative Specimen Type:
Plasma
Volume:
1 mL
Minimum Volume:
0.5 mL
Collection Container:
Lavender K2EDTA Tube
Transport Temperature:
Frozen, critical
Collection Information:
Centrifuge and separate plasma from cells ASAP or within 2 hours of collection. Transfer plasma to a standard aliquot tube.

Changes to Reference Ranges
Male
6 Months to 6 Years:
500 – 1700 pg/mL
7 to 9 Years:
522 – 1682 pg/mL
10 to 12 Years:
553 – 2071 pg/mL
13 to 15 Years:
485 – 2468 pg/mL
16 to 17 Years:
276 – 1546 pg/mL
18 to 29 Years:
238 – 1019 pg/mL
30 to 39 Years:
225 – 936 pg/mL
40 to 49 Years:
182 – 801 pg/mL
50 to 59 Years:
161 – 737 pg/mL
60 to 69 Years:
132 – 752 pg/mL
70 to 99 Years:
118 – 776 pg/mL
July 2022: Test Delay – Testosterone, Total and Free, Serum (TFTEST)
Immediate Test Change
Test Delay – Testosterone, Total and Free, Serum (TFTEST)
Delayed Test
Testosterone, Total and Free, Serum (TFTEST)
Send-out Testosterone, Total and Free, Serum (TFTEST) testing performed at Mayo Clinic Laboratories is currently delayed for approximately three weeks.
At this time, Mayo Clinic Laboratories does not have an estimate for when turnaround time will return to the expected value of four to six days.
Note:
Specimens are stable for 60 days when frozen.
Alternative Tests
Bioavailable Testosterone, SHBG, Adult Male (BTESTO)
In-house test for total testosterone by immunoassay and calculated bioavailable testosterone for adult males.
Bioavailable Testosterone/SHBG, Female & Child (BTSTFC)
Send-out test for total testosterone by LC-MS/MS and calculated bioavailable/free testosterone for children and adult females.
July 2022: Changes to Sulfonylurea Hypoglycemics (SULFON)
Special Communication
July 2022: Changes to Sulfonylurea Hypoglycemics (SULFON)
Effective July 5, 2022
Sulfonylurea Hypoglycemics (SULFON)
Changes to Specimen Requirements
Specimen Type:
Serum
Volume:
1 mL
Minimum Volume:
0.3 mL
Collection Container:
Red (Serum) No Additive Tube
Transport Temperature:
Frozen
Additional Info:
Centrifuge and aliquot ASAP or within 2 hours of collection.
Separate specimens must be submitted when multiple tests are ordered.

Alternative Specimen
Specimen Type:
Plasma
Volume:
1 mL
Minimum Volume:
0.3 mL
Collection Container:
Grey Hemogard, Sodium Fluoride/Potassium Oxalate Tube
Transport Temperature:
Frozen
Additional Info:
Centrifuge and aliquot ASAP or within 2 hours of collection.
Separate specimens must be submitted when multiple tests are ordered.
Changes to Reference Ranges
Refer to Report
- Rosiglitazone
- Chlorpropamide
- Glimepiride
- Glipizide
- Pioglitazone
- Glyburide
- Nateglinide
- Repaglinide
- Tolazamide
- Tolbutamide
Changes to Test Build
Add:
- Rosiglitazone
- Pioglitazone
Remove:
- Acetohexamide
June 2022: Changes to Testosterone, Total and Free, Serum (TFTEST)
Special Communication
June 2022: Changes to Testosterone, Total and Free, Serum (TFTEST)
Effective June 30, 2022
Testosterone, Total and Free, Serum (TFTEST)
Changes to Specimen Requirements
Specimen Type:
Serum
Volume:
2.5 mL
Minimum Volume:
1 mL
Collection Container:
Red (Serum) No Additive Tube
Transport Temperature:
Refrigerated

Changes to Reference Ranges
Male
1 to 8 Years:
<0.13 ng/dL
9 Years:
<0.13 – 0.45 ng/dL
10 Years:
<0.13 – 1.26 ng/dL
11 Years:
<0.13 – 5.52 ng/dL
12 Years:
<0.13 – 9.28 ng/dL
13 Years:
<0.13 – 12.6 ng/dL
Female
1 to 15 Days:
<0.13 – 0.25 ng/dL
16 to 364 Days:
Values decrease gradually from newborn (<0.13 – 0.25 ng/dL) to prepubertal levels.
*Citation: J Clin Endocrinol Metab 1973;36(6):1132-1142.
1 to 4 Years:
<0.13 ng/dL
5 Years:
<0.13 ng/dL
6 Years:
<0.14 ng/dL
7 Years:
<0.13 – 0.23 ng/dL
8 Years:
<0.13 – 0.34 ng/dL
9 Years:
<0.13 – 0.46 ng/dL
10 Years:
<0.13 – 0.59 ng/dL
11 Years:
<0.13 – 0.72 ng/dL
12 Years:
<0.13 – 0.84 ng/dL
13 Years:
<0.13 – 0.96 ng/dL
14 Years:
<0.13 – 1.06 ng/dL
15 to 18 Years:
<0.13 – 1.09 ng/dL
19 Years:
<0.13 – 1.08 ng/dL
20 to 24 Years:
<0.13 – 1.08 ng/dL
25 to 29 Years:
<0.13 – 1.06 ng/dL
30 to 34 Years:
<0.13 – 1.03 ng/dL
35 to 39 Years:
<0.13 – 1.00 ng/dL
40 to 44 Years:
<0.13 – 0.98 ng/dL
45 to 49 Years:
<0.13 – 0.95 ng/dL
50 to 54 Years:
<0.13 – 0.92 ng/dL
55 to 59 Years:
<0.13 – 0.90 ng/dL
60 to 64 Years:
<0.13 – 0.87 ng/dL
65 to 69 Years:
<0.13 – 0.84 ng/dL
70 to 74 Years:
<0.13 – 0.82 ng/dL
75 to 79 Years:
<0.13 – 0.79 ng/dL
80 to 84 Years:
<0.13 – 0.76 ng/dL
85 to 89 Years:
<0.13 – 0.73 ng/dL
90 to 94 Years:
<0.13 – 0.71 ng/dL
95 to 100 Years:
<0.13 – 0.68 ng/dL
May 2022: Immediate Test Discontinuation – Giardia lamblia IgG, IgA, IgM (GIAGAM)
Immediate Test Change
Immediate Test Discontinuation: Giardia lamblia IgG, IgA, IgM (GIAGAM)
Giardia lamblia IgG, IgA, IgM (GIAGAM)
Reason for Discontinuation:
Discontinued by Vendor
Alternative Test:
Cryptosporidium & Giardia Antigens by EIA (OVAPSC)
