Specimen Collection & Transport
Bone Marrow Biopsy Consultation Kit Instructions
Bone marrow aspirates and biopsies are performed in the evaluation of patients with suspected hematologic disease or hematolymphoid disorders.
The assessment of bone marrow samples by a staff hematopathologist requires detailed microscopic examination in conjunction with other ancillary testing, including flow cytometry, cytogenetics, and other molecular studies.
The best value for the patient is achieved when the treating physician provides pertinent clinical information, an adequate specimen is obtained, and samples are packed and shipped properly. Our Bone Marrow Biopsy Consultation Kit provides the necessary materials to ensure specimen integrity during shipment to our laboratories.
Specimen Collection & Transport
Overview
Bone marrow aspirates and biopsies are performed in the evaluation of patients with suspected hematologic disease including anemia, other cytopenias, leukemia, lymphoma, or other hematolymphoid disorders. The procedure is requested by the treating physician, usually a hematologist-oncologist, and the samples are evaluated by hematopathologists.
The assessment of bone marrow samples requires detailed microscopic examination in conjunction with other ancillary testing. Commonly used ancillary tests include flow cytometry, cytogenetics, and other molecular studies.
Clients may request a Comprehensive Bone Marrow Analysis, which includes microscopic examination and cytogenetics in all cases. Additional flow cytometry, immunohistochemistry, and/or molecular testing will be performed as directed by the hematopathologist after microscopic review. Alternatively, each individual diagnostic test may be ordered separately.
The best value for the patient is achieved when the treating physician provides pertinent clinical information, an adequate specimen is obtained, and samples are packed and shipped properly. Our Bone Marrow Biopsy Kit provides the necessary materials to ensure specimen integrity during shipment to our laboratories.

Requisition
Prior to sample collection, the treating physician should obtain and complete a Hematopathology Requisition.
Please ensure that each section is completed and that the contact information for the ordering physician is provided.

Specimen Requirements
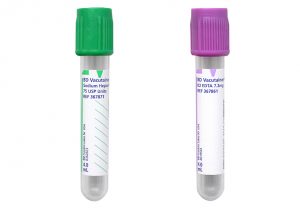
Aspirate
The initial bone marrow aspirate sample should be collected without anticoagulant and used to create 5-10 long smears. The leftover aspirate material is allowed to clot in the syringe (see Clot). Additional aspirate may be collected into an appropriate anticoagulant for ancillary testing.
For a Comprehensive Bone Marrow Analysis, the following samples are required:
• Flow cytometry: 4 mL in a sodium heparin tube
• Cytogenetics/FISH: 2–3 mL in a sodium heparin tube.
• Molecular studies: 3–4 mL in a K2EDTA tube.

Clot
The leftover aspirate without anticoagulant will clot within the syringe. Once clotted, remove from the syringe and place into one of the formalin containers provided.

Biopsy
An optimal trephine core biopsy should be at least 1–2 cm in length. Make touch preps by gently tapping the biopsy several times on a slide.
Place the biopsy into the other formalin container provided.
Shipping Preparation
1. When slides are labeled and dry, place them into the slide holders provided.

2. Liquid aspirate can be placed into the K2EDTA or sodium heparin tubes in the kit as needed.
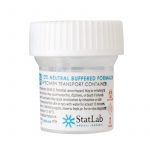
3. Place the clot and biopsy specimens into separate formalin containers.
4. Label all specimen parts with two of three patient identifiers:
- Patient name
- Date of birth
- Social security number
5. Place all specimens into the appropriate openings in the grey foam insert.

6. Place the foam insert into the provided sealable biohazard bag.

7. Secure the foam insert with the specimens into the white shipping box.

8. Place the completed Hematopathology requisition and CBC report in the Laboratory Pak.

9. Seal the specimens into the Laboratory Pak.

10. Affix the provided shipping label to the Laboratory Pak.
- Ship the specimen Next-Day Air to:
Cleveland Clinic Laboratories
L15 – Pathology & Laboratory Medicine
2119 E 93rd Street
Cleveland, OH 44106

Laboratory Contact Information
Manual Hematology Laboratory
216.444.2507
Contact the Manual Hematology Laboratory for assistance with technical shipping and handling questions.
Hematopathology Secretary
216.636.0186
Contact the Hematopathology Secretary to arrange to speak with a hematopathologist regarding medical pathology questions, including specific tests offered, their indications, and interpretations.
