Technical Brief:
M. tuberculosis Complex versus Non-Tuberculous Mycobacteria by Polymerase Chain Reaction (PCR) on Smear-Positive Specimens
Test Name
MTB Complex vs NTM by PCR on Smear Positive Specimens (TBPCR)
CPT Codes
87551
Methodology
Polymerase Chain Reaction (PCR)
Turnaround Time
7 days
Specimen Requirements
Specimen Type:
Smear-positive bronchoalveolar lavage (BAL)
Volume:
5 mL
Minimum Volume:
1.5 mL
Collection Container:
Sterile specimen container
Transport Temperature:
Frozen
Specimen Type:
Smear-positive sputum
Volume:
1 mL
Collection Container:
Sterile specimen container
Transport Temperature:
Frozen
Specimen Type:
Smear-positive pleural fluid
Volume:
5 mL
Collection Container:
Sterile specimen container
Transport Temperature:
Frozen
Alternative Specimen
Specimen Type:
Smear-positive tissue
Volume:
3 cubic mm
Collection Container:
Sterile specimen container
Transport Temperature:
Ambient
Stability
Ambient:
Unacceptable
Refrigerated:
Unacceptable
Frozen:
Resp. – 1 year if frozen within 72 hours
Tissue – 2 years if frozen within 24 hours
Additional Information
Background Information
Mycobacterium tuberculosis infects one-third of the world’s population and is the leading cause of death due to any infectious agent worldwide. The incidence of non-tuberculous mycobacteria (NTM) infections is increasing, and NTM isolates now are more common in the United States than M. tuberculosis. Strict isolation is required under the Centers for Disease Control and Prevention guidelines for all patients suspected of having tuberculosis; isolation is not required for patients infected with NTM. Treatment of tuberculosis and NTM also differs.
The ability to rapidly and accurately distinguish M. tuberculosis from NTM has significant clinical implications. This information should dictate appropriate infection control measures and guide the selection of appropriate antimicrobial therapy. The LightCycler system (Roche Diagnostics, Indianapolis, Ind.) combines real-time PCR with fluorogenic hybridization probes using fluorescence resonance energy transfer probes. This assay achieves rapid PCR results and has high sensitivity and specificity for the majority of clinically relevant mycobacteria, including M. tuberculosis, when smear-positive specimens are tested. Melting curve analysis performed by the LightCycler allows for differentiation of M. tuberculosis from NTM.
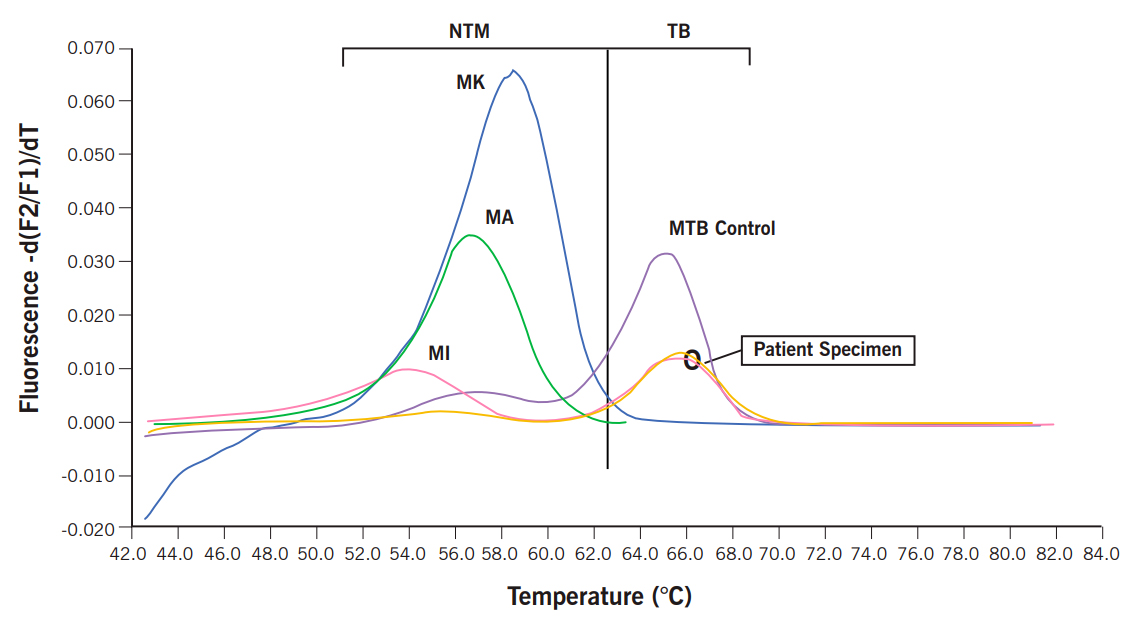
Clinical Indications
Detection and differentiation of M. tuberculosis from NTM on smear-positive specimens.
Culture for Mycobacterium spp. should be performed on all specimens ordered for acid-fast bacilli because of the possibility of dual mycobacterial infections and to have the isolate available for susceptibility testing if appropriate.
Interpretation
Results are reported qualitatively as positive or negative for M. tuberculosis, and positive or negative for NTM.
Limitations
This assay, as well as commercially-available assays, is insensitive when smear-negative specimens are tested.
This assay has suboptimal sensitivity for some of the rapidly growing Mycobacterium species and M. xenopi.
Methodology
The LightCycler FastStart DNA Master Hybridization Probe Kit (Roche) is used in conjunction with broad-range mycobacterial PCR primers and specially designed FRET hybridization
probes. Rapid-cycle PCR and post-amplification melt curve analysis are performed in the LightCycler system.
References
1. Shrestha NK, Tuohy MJ, Hall GS, Reischl U, Gordon SM, Procop GW. Detection and Differentiation of Mycobacterium tuberculosis and nontuberculous mycobacterial isolates by real-time PCR. J Clin Microbiol. 2003;41:5121-6.

