Special Communication
Changes To COMPC1, COMP3A, & COMP4A Testing
Complement C 1 (COMPC1)
Volume:
1 mL, serum
Minimum Volume:
0.25 mL
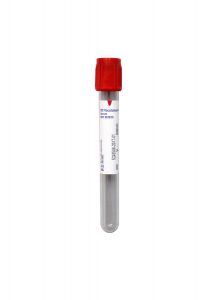
Collection Container:
Red BD Hemogard™ Serum Tube (No Additive)
Collection Instructions:
Allow blood to clot for 20 – 60 minutes at room temperature (or 37 °C), then centrifuge. Aliquot serum into a standard aliquot tube, then freeze the specimen immediately on dry ice or at -70 °C.
Transport Temperature:
Critical Frozen – send the frozen specimen via Priority Overnight in a well-insulated container on dry ice.
Reference Range
• 116,373 – 264,072 units/mL
Complement Component Level 3A (COMP3A)
Volume:
1 mL, plasma
Minimum volume:
0.5 mL
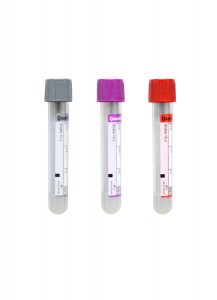
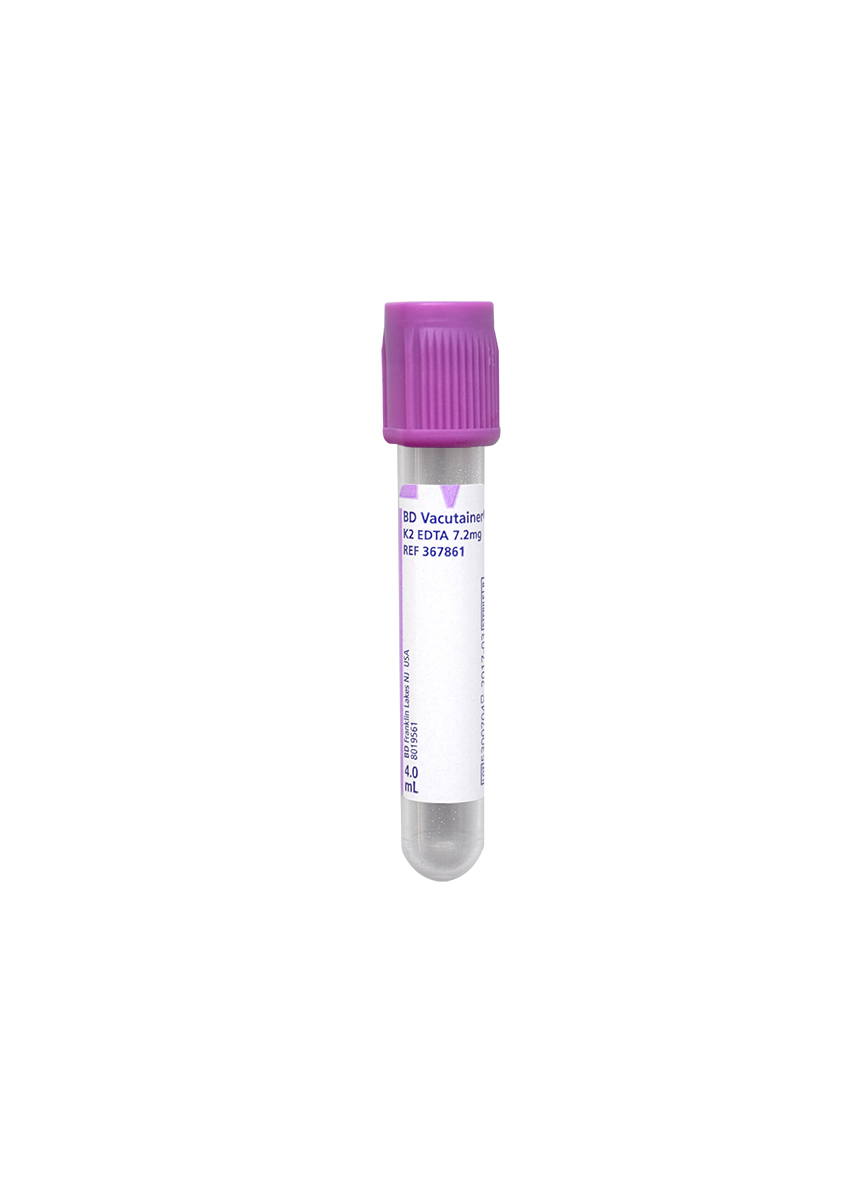
Collection Container:
Lavender BD Hemogard™ K2EDTA Tube
Collection Instructions:
Mix well. Centrifuge the specimen at room temperature within 30 minutes of collection, then transfer the plasma to a standard aliquot tube. Immediately freeze the specimen on dry ice or at -70°C.
Transport Temperature:
Critical Frozen – send the frozen specimen via Priority Overnight in a well-insulated container on dry ice.
Reference Range
• 0 – 780 ng/mL
Complement Component Level 4A (COMP4A)
Volume:
1 mL, plasma
Minimum volume:
0.5 mL
Collection Container:
Lavender BD Hemogard™ K2EDTA Tube
Collection Instructions:
Mix well. Centrifuge at room temperature within 30 minutes of collection, then transfer plasma to a standard aliquot tube and freeze immediately. Plasma may be frozen at -20°C then transferred to dry ice for shipment within 6 hours or immediately frozen on dry ice/at -70°C or below.
Transport Temperature:
Critical Frozen – send the frozen specimen via Priority Overnight in a well-insulated container on dry ice.
Reference Range
• 0 – 2,830 ng/mL
Any additional information will be included in a future Technical Update. If you have any questions about these changes, please contact Client Services for assistance.