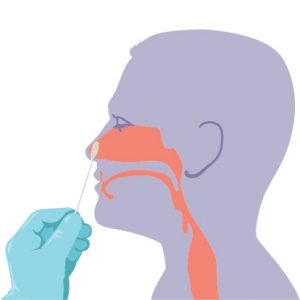
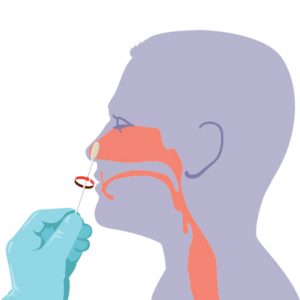
CAP Statement on the Current Role of Serologic Testing for SARS-CoV-2
May 13, 2020 | From the College of American Pathologists
Understanding COVID-19 & Antibody Testing
May 7, 2020 | From Cleveland Clinic’s Health Essentials:
We’ve accepted the harsh realities of COVID-19 and we’ve adjusted our lives accordingly. And yet, we still have so many unanswered questions like why has COVID-19 taken such a detrimental toll, and where are we in the process of finding a cure?
To get a better idea of where science is headed, Cleveland Clinic CEO and President Tom Mihaljevic, MD interviews Serpil Erzurum, MD, Chair of the Lerner Research Institute.
COVID-19 Serologic Testing: FAQS and Caveats
April 29, 2020 | From the Cleveland Clinic Journal of Medicine’s COVID-19 Curbside Consults:
There has been an immense amount of tangential discussion regarding the potential usefulness of serologic testing for COVID-19 recently. Although serologic testing has never been routinely used for diagnosing infections with “respiratory viruses”, such as influenzae, parainfluenzae, respiratory syncytial viruses, adenoviruses, or metapneumovirus, questioning its usefulness during the current outbreak has relevance.
That being said, during global epidemics of SARS, MERS, and H1N1 influenza, serology was never used in routine diagnostics. However, given the pandemic status of COVID-19 and the shortage of nucleic acid detection kits and/or swabs in certain areas, it raises the prospect of resorting to serology as an alternative to directly testing for the presence of the virus. The Infectious Diseases Society of America (IDAS) has recently issued a clear statement on COVID-19 serology.