Special Communication
Unacceptable Specimen Transport Media for COVID-19 Testing
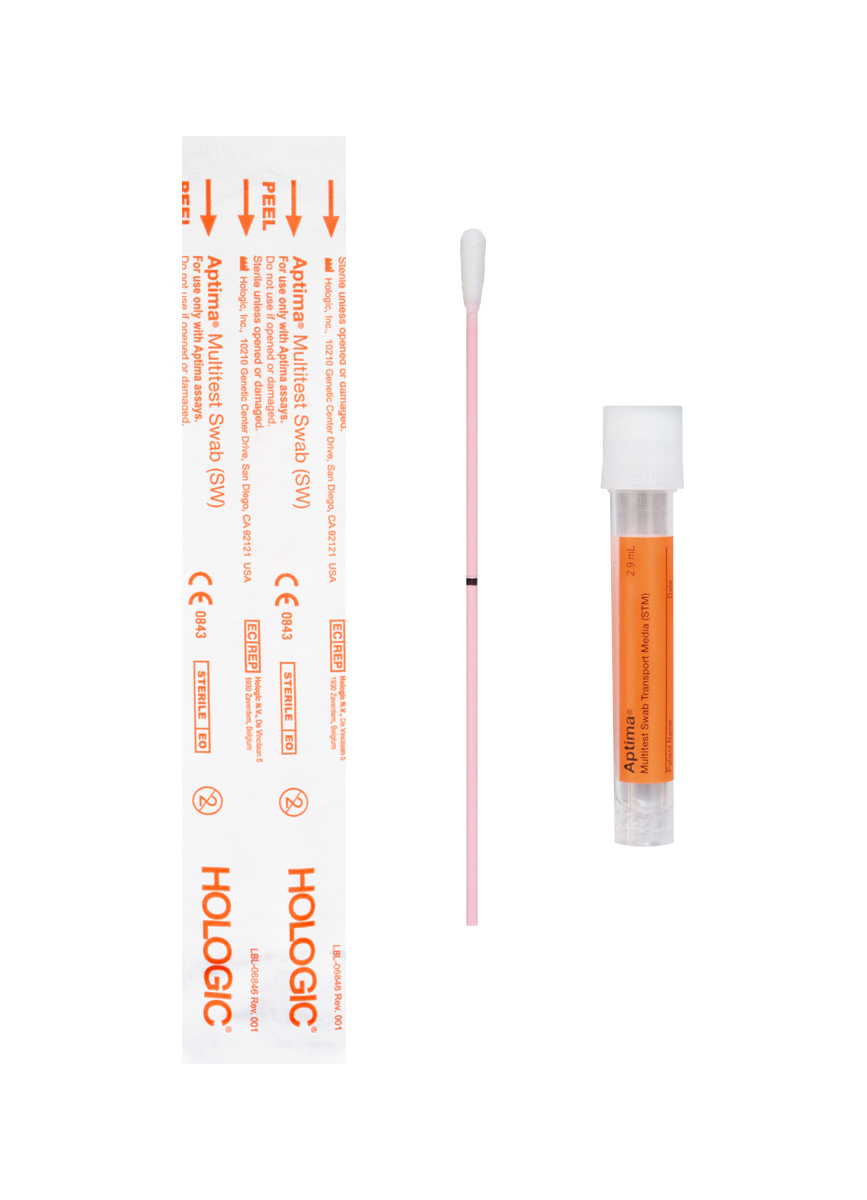
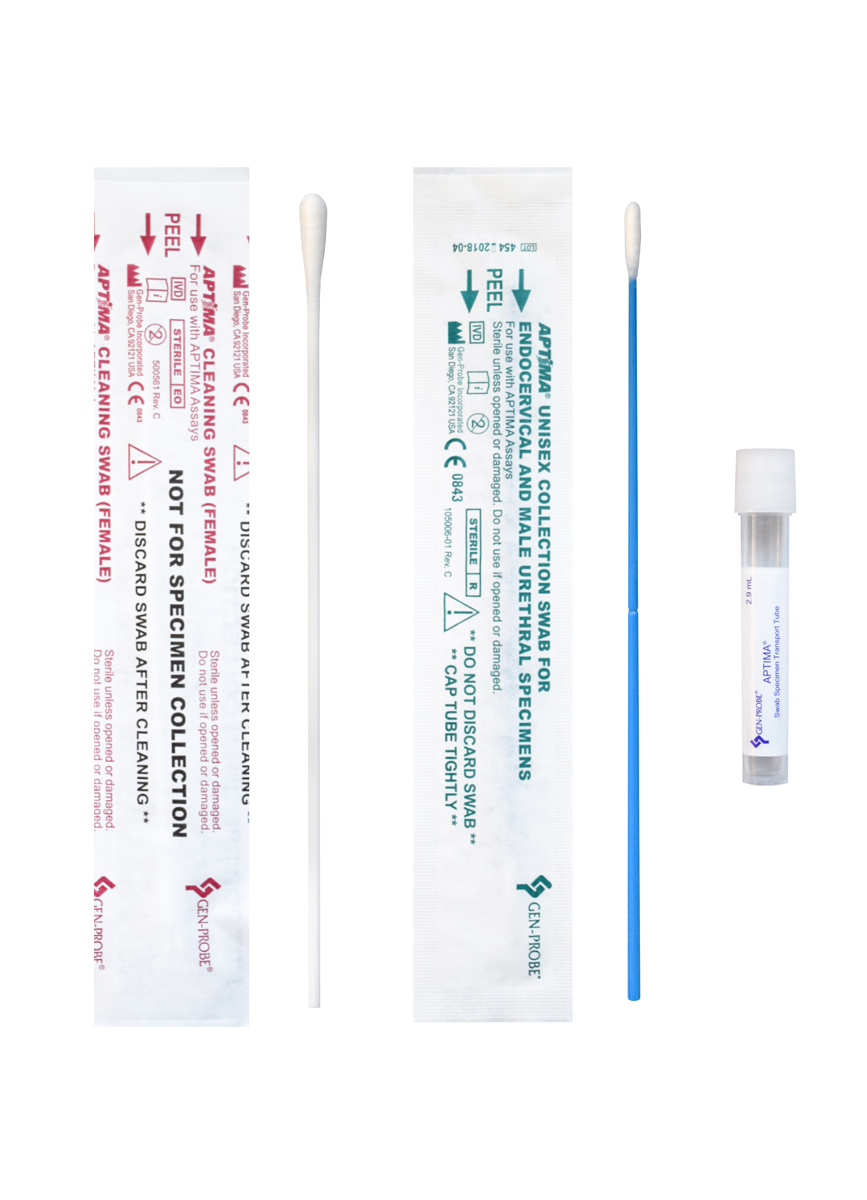
Due to incompatibilities with our COVID-19 testing laboratory equipment, Cleveland Clinic Laboratories has determined that specimens in the following media are not acceptable for COVID-19 testing performed by CCL:


The following specimen types are accepted by CCL for COVID-19 testing:




Universal Transport Media (UTM), 3 mL
Viral Transport Medium (VTM), 3 mL
Saline Transport Media, 3 mL
Sterile Container*, 3 mL
Specimen Type:
• Nasopharyngeal (NP) swab – preferred
• Nasal (anterior nares) swab
• Oropharyngeal (OP) swab
Specimen Type:
• Nasopharyngeal (NP) swab – preferred
• Nasal (anterior nares) swab
• Oropharyngeal (OP) swab
Specimen Type:
• Nasopharyngeal (NP) swab – preferred
• Nasal (anterior nares) swab
• Oropharyngeal (OP) swab
Specimen Type:
• Sputum
• Bronchoalveolar lavage (BAL)
*lower respiratory specimens only
Want More Information about COVID-19 Testing?
Review our Coronavirus 2019 Testing Overview for additional details, including collection instructions, required patient demographics, and more.
Need Collection & Transport Media?
If you are having difficulties obtaining appropriate COVID-19 specimen collection and transport supplies, please contact your CCL Account Manager.
Have a Question?
Please contact Client Services at 800.628.6818.