Test Name
D-Dimer (DDMER)
CPT Codes
85379
Methodology
Turbidimetric Immunoassay (TUI)
Turnaround Time
1 – 8 hours
Specimen Requirements
Volume:
2 mL
Minimum Volume:
1 mL
Specimen Type:
Plasma
Collection Container:
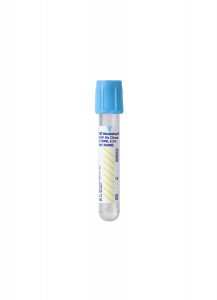
Light Blue Sodium Citrate Coagulation Tube
Transport Temperature:
Frozen
Reference Range
< 500 ng/mL FEU
Clinical Information
Useful in the evaluation of DIC and fibrinolytic abnormalities.
Useful in the evaluation of deep vein thrombosis.
A negative result (< 500 ng/mL) may be helpful in the exclusion of venous thrombosis.
Additional Information
Background Information
Venous thromboembolism is a serious medical problem that can escalate rapidly to a life-threatening situation in the form of pulmonary embolism. The annual incidence of pulmonary embolism in the United States is estimated at 100,000 to 300,000 cases.1
Symptoms of venous thromboembolism or deep vein thrombosis (DVT) include pain and swelling in the affected arm or leg and associated erythema, tenderness, and warmth in the affected limb and calf pain on foot dorsiflexion. Symptoms typically are unilateral and include dyspnea, pleuritic chest pain, hemoptysis, low-grade fever, and tachycardia.
The D-dimer has proven most useful in patients suspected of having a pulmonary embolism and who have a low pretest probability of disease. Use of the Wells Criteria has shown to be a reliable and reproducible means of determining this pretest probability. While DVT cannot be completely ruled out or confirmed with the Wells Criteria, it can help inform the interpretation of subsequent diagnostic tests and reduce the need for invasive testing.
An estimated 70 percent of patients presenting in the emergency room with symptoms of DVT do not have the disorder.1 Therefore, a means for rapidly and accurately differentiating between patients with DVT and those without is critical to defining subsequent appropriate therapy to prevent pulmonary embolism.
Several techniques have been proposed in the past decade for detecting small, deep thrombi, including CT venography, duplex scanning, and MRI. These techniques, while effective, are time-consuming, expensive, and, in the case of CT venography, expose patients to radiation.2
The D-dimer assay offers a rapid, non-invasive, relatively inexpensive in vitro method to rule out venous thromboembolism, DVT, or pulmonary embolism. The assay provides a quantitative measure of D-dimer.
- For suspected DVT, a D-dimer level below 500 ng/mL FEU has a Negative Predictive Value (NPV) of 99.2% and a sensitivity of 98.9 %.
- For suspected pulmonary embolism, a D-dimer below 500 ng/mL FEU has an NPV of 99.1% and a sensitivity of 97.8%.
The initial insult to the vein initiates the coagulation cascade. Fibrin produced during this process, together with platelet, forms the thrombus at the site of injury. During subsequent fibrinolysis, plasmin cleaves factor XIIIa–crosslinked fibrin into several intermediate forms, including D-dimer fragments. D-dimer fragments, resulting from terminal fibrin degradation, are composed of smaller segments of crosslinked fibrin and are produced only during fibrinolysis.
Under normal conditions, D-dimer is undetectable in the blood. Therefore, D-dimer is a marker for intravascular coagulation, and its presence in the blood is suggestive of thrombosis.
To make a definitive diagnosis of venous thromboembolism, DVT, or pulmonary embolism, patients with an elevated D-dimer should undergo additional testing such as ultrasonography, ventilation-perfusion lung scan, and chest computed tomography (CT).3
Clinical Indications
Testing rules out venous thromboembolism, DVT, or pulmonary embolism in the presence of risk factors and clinical symptoms.
Interpretation
Negative: D-dimer < 500 ng/ml
Limitations
D-dimer should not be used as a stand-alone test.
Elevated D-dimer may be due to recent surgery, trauma, or infection.
Elevated levels are also seen with liver disease, pregnancy, eclampsia, cardiovascular disease, and some cancers.
Suggested Reading
1. Bockenstedt P. D-Dimer in Venous Thromboembolism. N Engl J Med. 2003;349:1203-1204.
2. Stein PD, Matta F. Acute pulmonary embolism. Curr Probl Cardiol. 2010 Jul;35(7):314-76.
3. Salaun PY, Couturaud F, LE Duc-Pennec A, Lacut K, LE Roux PY, Guillo P, Pennec PY, Cornily JC, Leroyer C, LE Gal G. Non-invasive diagnosis of pulmonary embolism. Chest. 2010; Aug.19, e-pub ahead of print.