Immediate Test Change
TFTEST – Temporary Change in Performing Send-out Laboratory
November 4, 2021 – Update: Testosterone, Total and Free, Serum (TFTEST) has resumed being performed by Mayo Clinic Laboratories, the test’s original standard reference laboratory.
This change occurs automatically for TFTEST samples received by Mayo Clinic. Samples received prior to November 4, 2021, will be resulted from the temporary reference laboratory, Quest Diagnostics.
Refer to the details provided below for further information.
Testosterone, Total and Free, Serum (TFTEST) – Temporary Changes
Original Communication:
Beginning October 25, 2021, Testosterone, Total and Free, Serum (TFTEST) will be temporarily sent to an alternate reference laboratory (Quest Diagnostics).
This will occur automatically for new TFTEST samples received by Cleveland Clinic Laboratories.
Methodology
Methodology
The assays performed by both reference laboratories employ the same method, but there will be two main changes to the results:
1. Units of reporting for free testosterone will change from ng/dL to pg/mL.
Conversion factors:
- Testosterone, free (ng/dL) x 10 = Testosterone, free (pg/mL)
- Testosterone, free (pg/mL) / 10 = Testosterone, free (ng/dL)
2. Reference intervals for both free and total testosterone will be different.
- Mayo Medical Laboratories: complete reference intervals
- Quest Diagnostics: refer to the table below
Quest Diagnostics: Reference Intervals for Testosterone, Total (ng/dL)
Age
0-1 day
1-10 days
11-30 days
31 days – 2 months
3-4 months
5-6 months
7-11 months
12 months – 5 years
6-7 years
8-10 years
11 years
12-13 years
14-17 years
> 17 years
Male
17-61
<188
Not given
72-344
<202
<60
<17
<6
<26
<43
<261
<421
<1001
250-1100
Female
16-44
<25
Not given
<18
<13
<14
<12
<9
<21
<36
<41
<41
<41
2-45
Quest Diagnostics: Reference Intervals for Testosterone, Free (pg/mL)
Age
0-4 years
5-9 years
10-13 years
14-17 years
18-69 years
70-89 years
>89 years
Male
Not given
<5.4
0.7-52.0
18.0-111.0
35.0-155.0
30.0-135.0
Not given
Female
Not given
0.2-5.0
0.1-7.4
0.5-3.9
0.1-6.4
0.2-3.7
Not given
Specimen Requirements
Specimen Type
Serum
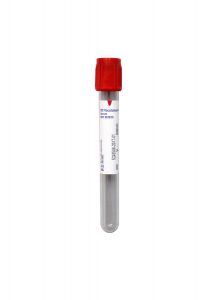
Collection Container
Red BD Hemogard™ Serum Tube (No Additive)
Volume
1.8 mL
Minimum Volume
0.9 mL
Turnaround Time
Turnaround Time
Turnaround time for samples sent to the temporary performing laboratory (Quest Diagnostics) is estimated at 10 to 14 days.